Every year in New Zealand around 4,000 people develop a blood clot in a vein. This article looks at venous thromboembolism – in particular why pregnancy puts women at increased risk – and how to help prevent, diagnose and treat pregnancy-associated venous blood clots.
By Tracey Woulfe
Reading the article Preventing and managing blood clots in pregnancy and undertaking this learning activity is equivalent to 60 minutes of professional development. This learning activity is relevant to the Nursing Council of New Zealand competencies: 1.1, 1.4, 2.2, 2.4, 2.7, 3.3 & 4.1.
Learning outcomes
Reading and reflecting on this article will enable you to:
- understand the pathophysiology of venous thromboembolism and Virchow’s triad
- apply this knowledge to understand why pregnancy increases the risk of venous thromboembolism (VTE) in this population
- identify additional risk factors associated with pregnancy associated venous thrombosis
- identify appropriate diagnostic and treatment options in pregnancy-associated VTE (PA-VTE).
Introduction
In New Zealand there are around 60,000 pregnancies per year1. It is estimated that between 30 and 140 of those births each year (0.5–2.2 per 1,000 deliveries2) will be affected by pregnancy-associated venous thromboembolism (PA-VTE).
The prevalence of PA-VTE may grow – with the trend for women to have babies later and the rising obesity levels – as risk factors include women being older than 35 and obesity.
Pregnancy-associated blood clots are recognised in the developed world as an important cause of maternal death and morbidity2,3. The 2015 Perinatal and Maternal Mortality Review Committee reported two out of 11 maternal deaths were directly attributed to PA-VTE1.
Venous thromboembolism: what is it?
Venous thromboembolism (VTE) is a collective term used to describe blood clots within the veins of the body.
Deep vein thrombosis (DVT) occurs predominantly in the legs; however, it can occur in the veins of the arm, abdomen or head. Any of these blood clots can break off, creating an embolus that travels to the lungs, where it is renamed a pulmonary embolus (PE) and is potentially life-threatening.
In pregnancy, the risk of developing VTE is four to five times greater than in non-pregnant women4,5, increasing in the post-partum period 20-fold6. Although the actual number of deaths remains low, the social and economic cost of associated morbidity is high4.
Midwifery researchers7 report that many women feel PA-VTE is trivialised by health professionals as only a short-term problem that is treatable with anticoagulants, while the long-term impact of the disease is not recognised. The known impact of PA-VTE on women during pregnancy or soon after birth can include or contribute to:
- post-thrombotic syndrome (PTS)
- pulmonary hypertension
- increased maternal ill health
- reduced quality of life.
In the general population, studies8,9 have found that patients who develop post-thrombotic syndrome (PTS) have a much poorer quality of life. Symptoms of post-thrombotic syndrome in the leg include chronic pain, redness, itching and swelling, and can lead to darkening of the skin and ulceration.
The psychosocial impact on the mother, her newborn and the family can also be considerable7. As some mothers struggle with the anxiety associated with PA-VTE, the likelihood of developing postnatal depression and bonding and attachment issues increases7.
The pathophysiology of VTE
In the mid-1800s, pathologist Rudolph Virchow described three factors associated with the development of blood clots, known today as Virchow’s triad10. These factors consist of:
- venous stasis
- a hypercoagulable state
- vascular wall injury.
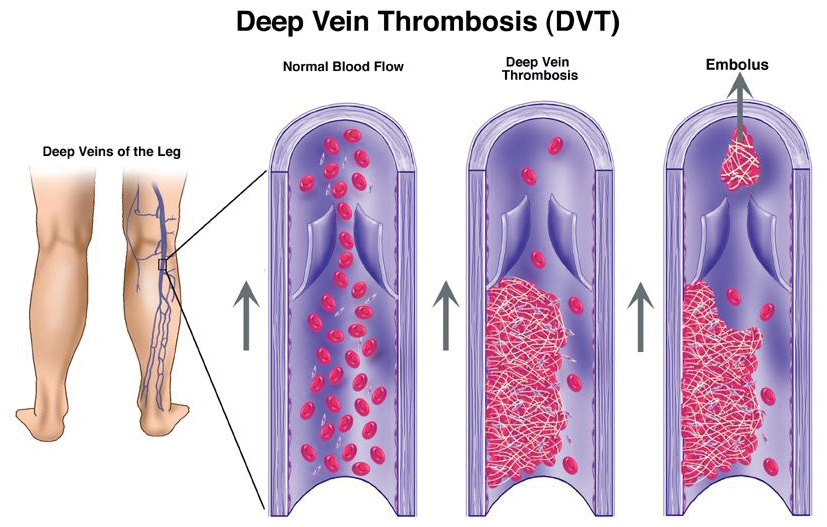
Venous stasis occurs in pregnancy due to increased venous dilation and the obstruction of venous return11. Obstruction is due to the compression of the left common iliac vein by the right common iliac artery and by the gravid uterus13. This compression of the left common iliac vein likely explains why 85 per cent of PA-DVT occurs in the left leg rather than the right leg13.
A hypercoagulable state develops because the body is preparing for the homeostatic changes that need to occur before delivery to prevent haemorrhage. Natural anticoagulants decrease in contrast to the pro-coagulant factors increasing11.
Vascular wall injury occurs as the growing foetus in-utero compresses the iliac artery onto the iliac vein, causing endothelial damage within the vein and activating the clotting cascade.
Venous stasis is believed to be the most clinically significant factor, and – when combined with either a hypercoagulable state or vascular wall injury – the risk of developing a blood clot is increased11. Only one of these factors needs to be present for a blood clot to occur. However, in pregnancy all three factors are present11,12.
The risk of PA-VTE is the same across all trimesters13, which may suggest that the hypercoagulable state is more important over this time. Additional endothelial damage, which occurs to the pelvic vessels during delivery3, may explain why up to a third of VTE occurs in the post-partum period, with 80 per cent of post-partum VTE occurring in the first three weeks following delivery. Data now suggests that this risk, albeit reduced, persists for up to 12 weeks after giving birth2,3,6.
Additional VTE risk factors
Additional risk factors in association with pregnancy increase the risk of developing PA-VTE. According to Australasian guidelines14, these include:
- previous VTE
- age >35
- high body mass index (BMI) >30kg/m2
- active medical illness
- smoking
- family history VTE
- immobility
- varicose veins
- multiparity (>2)
- multiple pregnancy
- pre-eclampsia
- assisted reproduction
- hyperemesis gravidarum (associated with dehydration and immobility)
- Caesarean section delivery (greater risk if an emergency vs elective)
- postpartum infection
- postpartum haemorrhage
- placental abruption.
Of note: inherited thrombophilia is absent from this list of additional risk factors and internationally this remains a debated issue. The presence or absence of known thrombophilia does not usually alter the acute or future management of VTE, therefore testing may create unnecessary anxiety for women15.
Thromboprophylaxis: preventing VTE
Risk assessment both antenatal and postnatal, plus the appropriate implementation of preventative measures, are all important steps in the prevention of PA-VTE5. However, there is a lack of large trials in pregnancy to provide the evidence from which to guide thromboprophylaxis decision-making.
In a review of international evidenced-based guidelines5, eight out of nine guidelines recommended women be assessed either pre-conception or early in their pregnancy for risk factors associated with VTE.
Three guidelines also recommend reassessment during pregnancy if a woman is hospitalised or develops additional complications such as pre-eclampsia. A single risk factor, such as a high BMI, is not in itself felt to be sufficient to warrant thromboprophylaxis. However, if a woman has more than one risk factor – or subsequently develops an additional risk factor such as immobility – then thromboprophylaxis is recommended5.
In New Zealand, when chemo-thromboprophylaxis is indicated, the standard of care is the low molecular weight heparin (LMWH), Clexane® 40mg subcutaneous, once daily. The Australasian guideline recommends Clexane 40mg twice a day if BMI >30kg/m2. 11
DIAGNOSING VTE IN PREGNANCY
The diagnosis of PA-VTE is challenging, as many of the normal pathophysiological changes that occur in pregnancy can be difficult to differentiate from the signs and symptoms of VTE, such as leg swelling and dyspnoea3,5.
Differential diagnosis may include musculoskeletal pain, chest infection or cellulitis. Making the diagnosis even more difficult is that approximately 80 per cent of DVTs remain clinically silent and woman do not present with the expected red, hot or swollen leg7. It is hypothesised that a delay in diagnosis in the post-partum period due to attention focusing on the newborn may explain why more women with proximal DVT in the post-partum period are more likely to develop PTS18.
Signs and symptoms (red flags) of pregnancy-associated deep vein thrombosis:
- Unilateral leg pain and swelling (more commonly in the left leg).
- Redness or discolouration of the limb.
- Warmth.
- Heaviness.
- Lower abdominal pain.
- Low-grade pyrexia.
Signs and symptoms (red flags) of pulmonary embolus:
- Dyspnoea.
- Chest pain.
- Haemoptysis.
Blood tests
An elevated D-dimer may indicate the formation of a new blood clot; however, as levels rise with other factors, such as trauma, surgery, cancer or pregnancy – especially in the last trimester and post-partum – testing is not recommended as it is not helpful in these situations12.
Clinical prediction rules
Outside of pregnancy, the Well’s score is a widely used and accepted predictive tool because of the high positive predictive value. To date there are no validated, structured prediction rules that can be applied in pregnancy15.
Objective testing
In mid to late pregnancy, DVT is 85 per cent more likely to occur in the left leg than the right leg13, largely due to the compression of the left iliac vein by the gravid uterus, as discussed earlier.
When compared with the non-pregnant population, it is also much more likely to occur proximally, with 72 per cent involving the veins in the pelvis in pregnancy as opposed to only 9 per cent outside of pregnancy3. This makes diagnosis by ultrasound scan (USS) challenging, as a negative scan from groin to ankle can miss an isolated pelvic vein clot, therefore clinicians and midwives should be advised to investigate further if high clinical suspicion exists of a DVT.
When a PE is suspected, a ventilation perfusion (VQ) scan is the preferred diagnostic scan in pregnancy13, as is it more likely to see smaller lung clots. Computed tomography pulmonary angiogram (CTPA) can miss up to 30 per cent of smaller lung clots due to the higher circulating blood volume in pregnancy; with total body water increasing up to eight litres in pregnancy19. Furthermore, radiation exposure to the breast tissue is significantly higher with CTPA; although if CTPA is the only choice then breast shields should be used to reduce the radiation exposure.
There is a low radiation risk to the foetus (<50 mGy) with either VQ scan or CTPA, which is far below the threshold linked to childhood malignancies6,15.
TREATMENT
Anticoagulants
Due to ethical issues associated with pregnancy research, there is a lack of pharmacokinetic data specific to pregnancy, and recommendations are based on observational studies and basic pharmacology principles19. In pregnancy, drug absorption usually decreases, whereas drug excretion increases, leading to lower plasma concentrations19.
Warfarin has been the mainstay of VTE treatment for over 50 years. However, its use in antenatal PA-VTE is usually contraindicated because of the known teratogenic effects on the foetus, along with the potential to cause foetal loss and bleeding2. The exceptions to this, outside of PA-VTE, are women at high risk of arterial vein thrombosis due to mechanical heart valves, where warfarin is more efficacious than low molecular weight heparin (LMWH) in preventing arterial thrombosis11.
As the foetus has low levels of clotting factors and is vitamin K-deficient at birth, therapeutic warfarin levels in the mother will likely over-anticoagulate the foetus, posing significant haemorrhagic risk if the mother delivers early11. Therefore, the use of antenatal warfarin in the context of PA-VTE is not recommended, and its use should only be considered in exceptional circumstances15.
New direct oral anticoagulants (DOACs) such as Pradaxa® or Xarelto® have recently been licensed for VTE treatment in New Zealand; however, there is no data to support their use in pregnancy. Direct oral anticoagulants could potentially be a suitable option in the post-partum period if the mother were not breastfeeding.
Currently the use of low molecular weight heparin (LMWH) is the only recommended anticoagulant treatment supported in international and local guidelines. In New Zealand we use Clexane®, which restricts the formation of new clots and provides time for the breakdown of an existing clot to occur13.
In New Zealand the accepted weight-based therapeutic dosing of Clexane® is either 1mg/kg twice a day or 1.5mg/kg once a day16. Anti-factor Xa levels, which measure the effect of LMWH, are generally used only if a woman has a second clot while on treatment, or in obese patients2.
Pregnancy increases renal blood flow by 60–80 per cent, with drug elimination increasing by 50 per cent due to an increase in the glomerular filtration rate19. Increased renal clearance and greater volume of distribution would indicate a need for higher dosing requirements18.
In reality, there remains a lack of data on once-daily versus twice-daily dosing2,3. The Sanofi datasheet16 suggests that twice-daily dosing may be beneficial in obese patients with pelvic vein clots, but refrains from giving any pregnancy-specific dosing guidelines.
Graduated compression stockings
Guidelines20 still recommend the use of graduated compression stockings for treating symptoms of PA-VTE, such as reducing oedema, along with initial leg elevation; however, they stipulate that the evidence to support their use in preventing post-thrombotic syndrome (PTS) is unclear. The ACCP guidelines21 do still suggest that graduated compression stockings may have some symptomatic benefit in the acute treatment stage.
MANAGEMENT
Antenatal
In the antenatal period, low molecular weight heparin (LMWH) is the preferred treatment option because the dose-response is more predictable than unfractionated heparin (UFH). The long-term use of UFH is also associated with a 30 per cent reduction in bone density (osteopenia), leading to an increase in osteoporotic fractures and a higher incidence of heparin-induced thrombocytopenia marked by a drop in platelets2,6.
The dosing of LMWH in the antenatal period is dependent on the size, location and timing of the DVT or PE.
Labour and delivery
All pregnant women should be advised to withhold LMWH at the first sign of labour, as the timing and amount of their last dose will affect whether or not they will be able to have an epidural22.
Post-partum
In woman with confirmed PA-VTE in the antenatal period it is recommended that post-partum anticoagulation should be recommenced as soon as haemostasis is achieved; usually six hours post-normal vaginal delivery and 12 hours post-caesarean section6, however please refer to your local guidelines as recommendations may differ.
New PA-VTE that occurs in the post-partum period can be managed the same way as a provoked DVT or PE outside of pregnancy with warfarin therapy, and potentially a direct oral anticoagulant if the mother is not breastfeeding. Choice and duration of anticoagulation will again depend on the size and location of the clot. If a woman was at high risk of bleeding, intravenous UFH would be indicated in the first instance before switching to a LMWH and establishing oral anticoagulation.
SYNTHESIS OF CURRENT BEST PRACTICE
The prevention, treatment, and management of PA-VTE is challenging due to the scarcity of high-quality evidence and data specific to pregnancy, complicated by both maternal and foetal concerns.
The evidence for the use of thromboprophylaxis to prevent PA-VTE is an extrapolation of data from studies involving non-pregnant participants, so identifying the right target population within pregnancy is challenging. There is consensus in the international guidelines5 that each woman needs to be individually assessed so an informed decision can be made collaboratively once the woman understands the balance of risk versus benefit.
No consensus exists for prophylactic dosing of LMWH in obese patients or in the treatment dosing of antenatal PA-VTE and further studies are required to address this issue. The use of direct oral anticoagulants may have a place in the post-partum treatment of PA-VTE in non-breastfeeding women.
Warfarin and LMWH are both safe to use in the post-partum period as neither passes through the breast milk. The choice of anticoagulation depends on factors such as size, location and timing of the DVT or PE, the required duration of treatment, and patient choice22. (It should be noted that in New Zealand Clexane® is only funded antenatally and for up to six weeks post-partum.) If anticoagulation treatment were indicated for longer in the post-partum period, oral anticoagulation would be the only option.
Diagnosis of VTE in pregnancy is complicated and challenging due to the normal physiological changes that occur in pregnancy, which can mirror a differential diagnosis and lead to a delay in timely investigation and treatment. Ultrasound scans remains the most convenient and accessible means of diagnosing DVT. The choice of lung scanning is more uncertain, with the decision often coming down to accessibility, clinician and patient preference, and local guidelines.
While there no longer appears to be a role for the use of graduated compression stockings in preventing PTS, these stockings might still provide symptomatic relief in some patients and should still be considered.
Summary
PA-VTE remains in many instances a preventable disease, provided patient risk factors are adequately assessed and appropriate interventions taken. As society changes, the prevalence of some risk factors for PA-VTE are increasing, such as increasing age and BMI, coupled with caesarean delivery.
Identifying risk factors, and implementing preventative measures, is achievable and would go a long way in reducing the social and economic cost inked to maternal death and morbidity. Empowering the mother through education and addressing her concerns early may reduce the anxiety associated with PA-VTE.
Download the learning activity here >>
Table 1: Bullying behaviours
Work-related
- Someone withholding information that affects your performance.
- Being ordered to do work below your level of competence.
- Having your opinions ignored.
- Being given tasks with unreasonable deadlines.
- Excessive monitoring of your work.
- Pressure not to claim something to which by right you are entitled.
- Being exposed to an unmanageable workload.
Person-related
- Being humiliated or ridiculed in connection with your work.
- Having key areas of responsibility removed or replaced with more trivial or unpleasant tasks.
- Gossip and rumours being spread about you.
- Being ignored or excluded.
- Having insulting or offensive remarks made about your personality, attitudes or private life.
- Hints or signals from others that you should quit your job.
- Repeated reminders of your errors or mistakes.
- Being ignored or facing a hostile reaction when you approach.
- Persistent criticism of your errors or mistakes.
- Practical jokes being carried out by people you don’t get along with.
- Having allegations made against you.
- Being the subject of excessive teasing and sarcasm.
- Being shouted at or being the target of spontaneous anger.
Physically intimidating
- Intimidating behaviours, such as finger-pointing, invasion of personal space, shoving, blocking your way.
- Threats of violence or physical abuse or actual abuse.
Source: Negative Acts Questionnaire – Revised7
Recommended resources
New Zealand VTE prevention programme – National Policy Framework (2012): This comprehensive document includes risk assessment tools and patient information: https://bit.ly/2GYVu2x
Clinical Pathway for Deep Vein Thrombosis: This two – page flowchart developed for the Auckland region includes the Wells’ Score: https://bit.ly/2Gvzr3M.
About the author
Tracey Woulfe RCompN PGDip MHSc is a thrombosis clinical nurse specialist at Waitemata District Health Board.
This article was peer reviewed by
Rhonda J Robertson RN RM BSc(Hons) PGCert CT PGDip(Mid), who is a clinical midwife educator at the Canterbury District Health Board and Daryl Pollock RN MN, who is a thrombosis/haemostasis clinical nurse specialist at MidCentral District Health Board.
References
- PERINATAL AND MATERNAL MORTALITY REVIEW COMMITTEE (2015). Eleventh annual report of the perinatal and maternal mortality review committee: Reporting mortality 2017. Health Quality and Safety Commission, Wellington.
- BATES S, MIDDLEDORP S & RODGER M et al (2016). Guidance for treatment and prevention of obstetric-associated venous thromboembolism. Journal of Thrombosis and Thrombolysis 41.
- GREER I A (2015). Pregnancy complicated by venous thrombosis. The New England Journal of Medicine 373(6).
- KOURLABA G, RELAKIS J et al (2016). A systematic review and meta-analysis of the epidemiology and burden of venous thromboembolism among pregnant women. International Journal of Gynecology and Obstetrics 132(1).
- OKOROCH E, AZONOBI I et al (2012). Prevention of venous thromboembolism in pregnancy: A review of guidelines 2000-2011. Journal of Women’s Health 21(6).
- MARSHALL A (2014). Diagnosis, treatment, and prevention of venous thromboembolism in pregnancy. Postgraduate Medicine 126(7).
- CARTER K & MUNRO J (2015). The shadow of venous thromboembolism. Midwives 18.
- KAHN S, SHBAKLO H et al (2008). Determinants of health-related quality of life during the 2 years following deep veins thrombosis. Journal of Thrombosis and Haemostasis 6(7).
- VAN KORLARR I, VOSSEN C et al (2004). The impact of venous thrombosis on quality of life. Thrombosis Research 114(1).
- KUMAR D, HANLIN E et al (2010). Virchow’s contribution to the understanding of thrombosis and cellular biology. Clinical Medicine & Research 8(3/4).
- MCLINTOCK C (2014). Thromboembolism in pregnancy: Challenges and controversies in the prevention of pregnancy-associated venous thromboembolism and management of anticoagulation in women with mechanical prosthetic heart valves. Best Practice & Research Clinical Obstetrics and Gynaecology 28.
- SIMCOX L, ORMESHER L et al (2015). Pulmonary thromboembolism in pregnancy: diagnosis and management. Breathe 11(4).
- BOURJEILY G, PAIDAS M et al (2010b). Pulmonary embolism in pregnancy. Lancet 375.
- MCLINTOCK C, BRIGHTON T et al (2012). Recommendations for the prevention of pregnancy-associated venous thromboembolism. Australian and New Zealand Journal of Obstetrics and Gynaecology 52(1).
- CHAN W, REY E & KENT N (2014). Venous thromboembolism and antithrombotic therapy in pregnancy. Journal of Obstetrics and Gynaecology Canada 36(6).
- SANOFI-AVENTIS (NZ) Ltd. (2015). New Zealand Datasheet. Retrieved from www.medsafe.govt.nz.
- WIK H, JACOBSEN A et al (2012). Prevalence and predictors for post-thrombotic syndrome 3 to 16 years after pregnancy-related venous thrombosis: a population-based, cross-sectional, case-control study. Journal of Thrombosis and Haemostasis 10.
- GANDAR E, CARRIER M & RODGER M (2014). Management of pregnancy associated venous-thromboembolism: a survey of practices. Thrombosis Journal 12(12).
- DAWES M & CHOWIENCZYK P (2001). Pharmacokinetics in pregnancy. Best Practice & Research Clinical Obstetrics and Gynaecology 15(6).
- ROYAL COLLEGE OF OBSTETRICIANS AND GYNAECOLOGISTS (2015). Reducing the risk of venous thromboembolism during pregnancy and puerperium. Green-top guideline No. 37a.
- KEARON C, AKL E et al (2016). Antithrombotic therapy for VTE disease. CHEST 149(2).
- CHANDRARAJAN L & NELSON-PIERCY C (2015). Risk of venous thromboembolism during pregnancy and birth. British Journal of Midwifery 23(9).
- TAPSON V (2008) Acute Pulmonary Embolism. New England Journal of Medicine 358.